| Meeting Brochure and registration form SMDM Homepage | |

|

|
|
||||
Methods: We used published studies on the efficacy, side effects, and cost of a sequence of HIV drug regimens under a standard of care (SOC) strategy (year 1: 93% efficacy, $14,000 regimen cost) compared to the PI/r strategy (83% efficacy, $12,000 regimen cost). Using a published Monte Carlo model of HIV disease (CEPAC), we projected life expectancy (LE), discounted quality-adjusted life expectancy (QALE), and discounted lifetime costs for each strategy for subjects at different CD4 count levels.
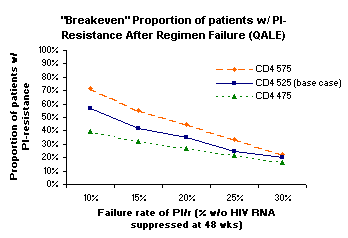 Results: Subjects receiving the PI/r strategy who at the time of first regimen failure have not developed PI-resistant HIV have a higher LE (28.3 vs. 27.6 years) and QALE (15.1 vs. 14.9 years) compared to SOC, as they gain an additional regimen without negative consequences for subsequent treatment options. The expected QALE of the PI/r strategy remains superior even when a large proportion of failures develop PI resistance: depending on the probability of PI/r regimen failure, 20-57% can develop PI resistance (see figure). Lifetime costs are $18,000-$38,000 lower for the PI/r strategy than SOC.
Results: Subjects receiving the PI/r strategy who at the time of first regimen failure have not developed PI-resistant HIV have a higher LE (28.3 vs. 27.6 years) and QALE (15.1 vs. 14.9 years) compared to SOC, as they gain an additional regimen without negative consequences for subsequent treatment options. The expected QALE of the PI/r strategy remains superior even when a large proportion of failures develop PI resistance: depending on the probability of PI/r regimen failure, 20-57% can develop PI resistance (see figure). Lifetime costs are $18,000-$38,000 lower for the PI/r strategy than SOC.
Conclusions: Simulation modeling identifies key trial endpoints and differences in perspective. With low PI/r failure rates, higher probabilities of PI resistance are acceptable for the population than may be clinically acceptable to subjects enrolling in the trial.
See more of Concurrent Abstracts I: Clinical Strategies or Guidelines
See more of The 28th Annual Meeting of the Society for Medical Decision Making (October 15-18, 2006)