Zefeng Zhang, MD, PhD1, Paul Kolm, PhD
1, John Spertus
2, Liping Zhao
1, Claudine Jurkovitz
1, Marion Kvasz
3, Richard Willke
3, and William S. Weintraub, MD
1. (1) Christiana Care Health System, Newark, DE, (2) Mid America Heart Institute, Kansas City, MO, (3) Pfizer Inc., New York, NY
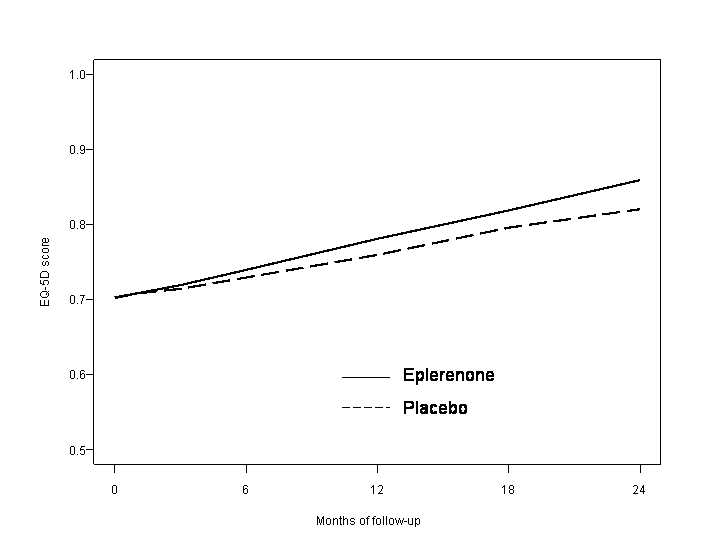
Purpose: The EPHESUS trial demonstrated the efficacy of eplerenone vs. placebo in terms of a reduction in morbidity and mortality endpoints in patients with heart failure (HF) after acute myocardial infarction (AMI). The purpose of this study was to compare EQ-5D trends of eplerenone and placebo patients over the follow-up period. Methods: The EQ-5D was administered to 2,280 (34%) of EPHESUS patients at baseline, and at 1, 3, 6, 12, 18, and 24 months following study entrance. Change in EQ-5D scores over the follow-up period was analyzed by linear mixed effects models. The analysis included gender and time-varying covariates, age and repeat hospitalizations. For the analysis, a repeat hospitalization was assumed to impact the subsequent EQ-5D score. Missing scores were assumed to be missing at random (MAR). Results: There was a significant treatment group by follow-up time interaction (p = 0.047) indicating a difference in trends. Trends of an increase in EQ-5D scores were significant for both groups, but eplerenone patients evidenced a greater increase over the follow-up period. As would be expected, increasing age and repeat hospitalizations had a negative effect on EQ-5D scores, and males had higher scores than females (p < 0.001). Conclusions: The EPHESUS trial demonstrated not only a benefit of eplerenone with respect to the major endpoints, but also a benefit in health status in patients with AMI complicating HF. Health status and quality of life have become important measures to include in clinical trials. Differences in health status scores between treatment groups as evidenced in the EPHESUS trial indicate the importance of obtaining such information to evaluate not only the impact of interventions on patients perceptions of health, but to provide estimates of quality adjusted life years in cost-effectiveness analyses.
See more of Poster Session II
See more of The 28th Annual Meeting of the Society for Medical Decision Making (October 15-18, 2006)

