|
||||
Monday, October 22, 2007
P2-42
CARDIOVASCULAR DISEASE RISK ASSOCIATED WITH AROMATASE INHIBITOR VS. TAMOXIFEN TREATMENT IN BREAST CANCER PATIENTS
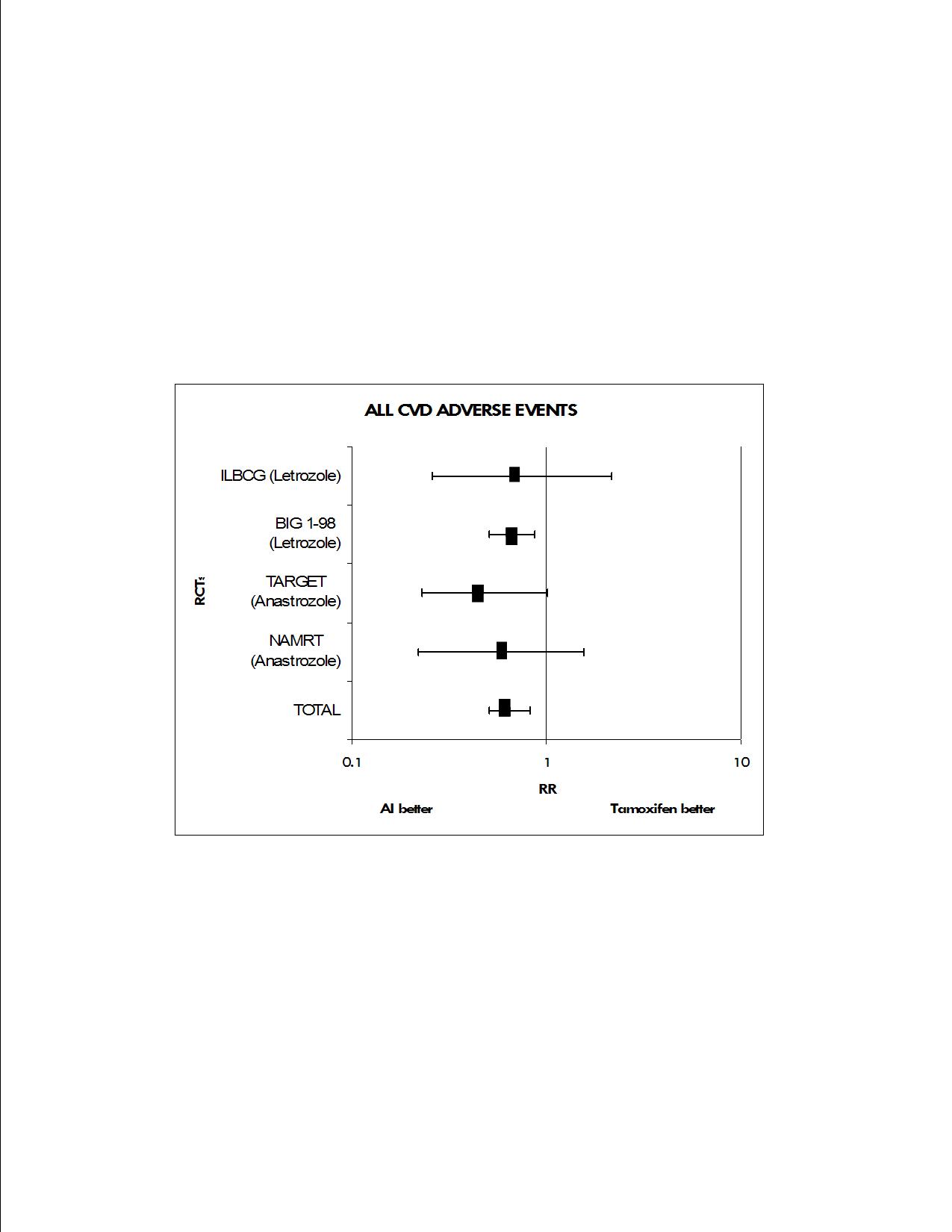
Method(s): We searched Pubmed/MEDLINE and EMBASE for randomized controlled trials (RCTs) directly comparing a third generation AI (including anastrozole, exemestane or letrozole) to tamoxifen. Only studies that reported the prevalence of CVD adverse effects in postmenopausal women were included. Studies were independently abstracted by 3 people for CVD adverse events, defined as venous thromboembolism (VTE, including pulmonary embolism and deep vein thrombosis), myocardial infarction, transient ischemic attack (TIA) or cerebral vascular accident (CVA). We pooled data using meta-analytic methods specifically the random effects model of DerSimonian and Laird.
Results: A total of 1,230 articles were identified by our search criteria; 4 studies (2 letrozole, 2 anastrozole) reported all three CVD endpoints. No exemestane RCTs met the cardiovascular outcomes criteria. The median age of participants was 64 years. Anastrozole and letrozole (combined) were associated with fewer CVD adverse events (RR 0.65) compared to tamoxifen. Alone, anastrozole treatment was associated with a decreased risk of CVD adverse events (RR 0.52) compared to tamoxifen treatment, while letrozole was also associated with CVD adverse events (RR .67).
Conclusion: Our findings suggest that anastrozole and letrozole are associated with a decreased risk of CVD adverse events compared to tamoxifen. Understanding how these AIs differentially influence cardiovascular risk may improve patient care.